+ 49%
Increase in Ocean Biodiversity, enabling efficient life-spans
500,000
5,000,000
How does this actually work?
Electrochemical Systems
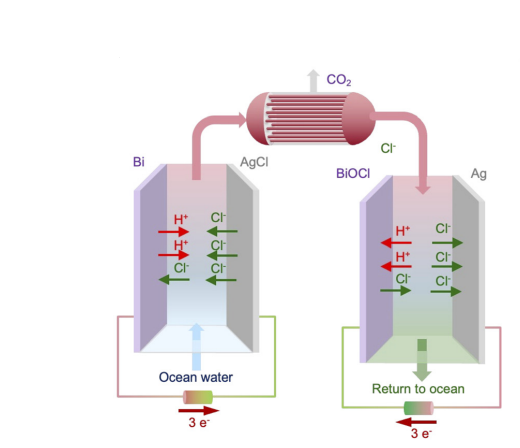
The method described for removing carbon dioxide from ocean water involves using an electrochemical system with bismuth and silver electrodes that can capture and release chloride ions. The difference in reaction stoichiometry between the two electrodes enables an electrochemical system architecture for a chloride-mediated electrochemical pH swing, which can be leveraged to remove CO2 from ocean water without costly bipolar membranes effectively.
Operating BismuthSystems
We utilize two silver-bismuth systems operating in tandem in a cyclic process. One system would acidify the ocean water, and the other would regenerate the electrodes through the alkalization of the treated stream. This would allow for CO2 to be continuously removed from simulated ocean water with a relatively low energy consumption of 122 kJ mol−1, and high electron efficiency. Ensuring effective remocal of carbon.
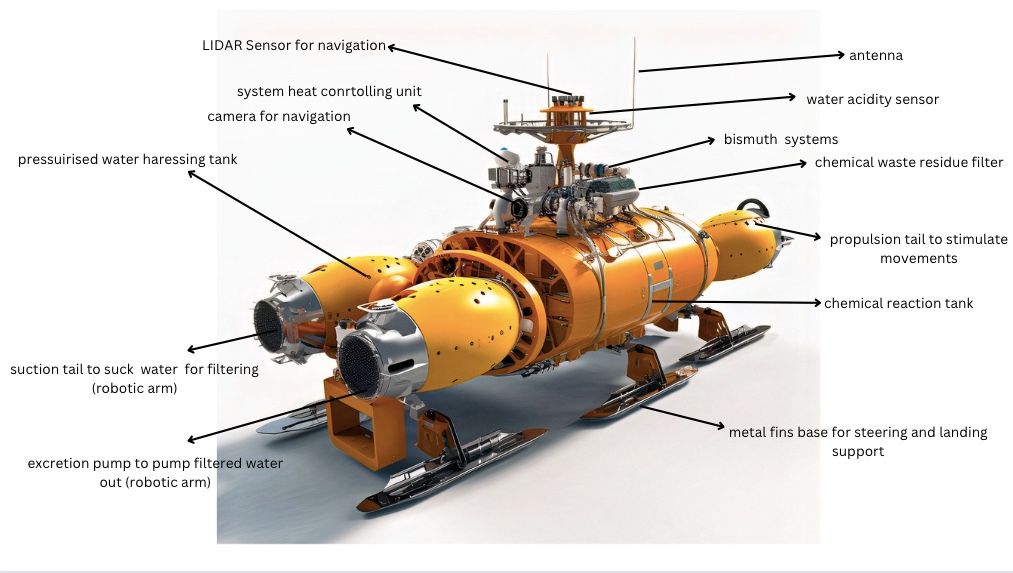
AUV and ROV Integration
The design includes a system for deploying and controlling the electrochemical system in the ocean. This could involve autonomous underwater vehicles (AUVs) or remotely operated vehicles (ROVs) to transport and operate the electrochemical system. The AUV or ROV is equipped with an electrochemical system and the necessary sensors and control systems to monitor and adjust the operation of the system.
A technology-first approach to save our oceans
Why H2OAQUATICS makes sense!
The traditional methods of addressing ocean acidification, such as reducing carbon emissions and promoting the growth of seaweed forests, have proven to be insufficient in curbing the effects of ocean acidification. Thus, highlighting the need for change.
According to the National Oceanic and Atmospheric Administration (NOAA), ocean acidification is happening at a rate faster than any time in the past 300 million years. The current rate of ocean acidification is causing serious harm to marine life and ecosystems, with shell-forming organisms such as oysters and clams being particularly vulnerable.
The proposed electrochemical system can remove CO2 from seawater with relatively low energy consumption and high electron efficiency. The system requires only 122 kJ mol−1, which is significantly lower than other carbon capture and storage technologies. This means that the proposed system could be more cost-effective than other methods of addressing ocean.
The proposed electrochemical system has the potential to remove CO2 from ocean water continuously, unlike other methods that may be limited by factors such as weather conditions and the availability of suitable locations for seaweed forests or carbon storage facilities.
The proposed electrochemical system can be scaled up to suit different levels of carbon capture needs, from small-scale applications to large industrial installations. In the near future, this scalability gives us the heads up, that our solution IS THE ANSWER.
In terms of specific numbers, the proposed system's energy efficiency of 122 kJ mol−1 compares favorably to other carbon capture technologies that typically require 250-350 kJ mol−1. Additionally, the system's scalability makes it a versatile solution for addressing ocean acidification on a wide scale.
We Present...
Our Product
The Future of Ocean Automation
Once the robot arrives at the target location, it must deploy the electrochemical system into the ocean. This could involve the use of robotic arms or other mechanisms to position the electrodes in the water. The robot would also need to ensure that the electrochemical system is secured in place to prevent it from drifting away.
Impacts: Efficient Carbon Capture, De-Acidified Water, increase in Biodiversity lifespans.
Function
Mechanisms
The mechanisms that take place within the AOV/ROV with the ocean structures.
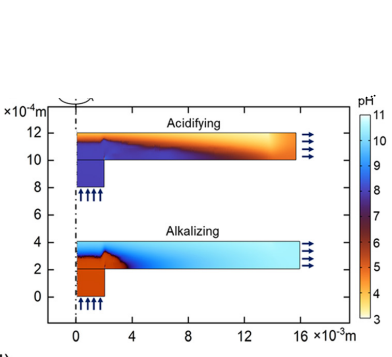
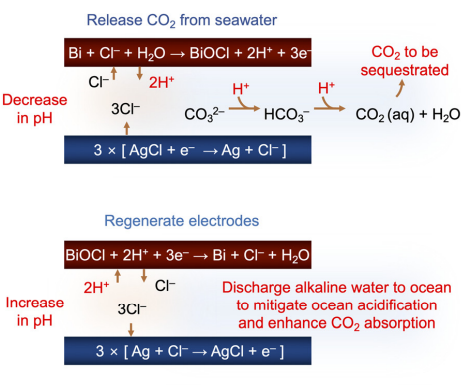
Acidifying and Alkalizing
A depiction of the process of carbon capture through our created model, forecasted on ocean waters.
Molecular Structures
Through our process of relseasing CO2 to Regenerating electrodes, the scientific molecular sequestration process readily releases CO2 from the water.V

Don't miss out
Get updates on our operations and join us by being a part of this EPIC journey :)
Do we really want to risk endangering the sole source of 70% of the world's oxygen... JOIN THE CAUSE
H2OAQUATICS
Copyright © H@OAQUATICS